what alkene is required to make 3-methyl-1-butanol using the hydroboration-oxidation reaction?
Hydroboration-Oxidation of Alkenes
- Folio ID
- 889
Hydroboration-Oxidation is a two step pathway used to produce alcohols. The reaction proceeds in an Anti-Markovnikov manner, where the hydrogen (from BH3 or BHR2) attaches to the more substituted carbon and the boron attaches to the least substituted carbon in the alkene bouble bond. Furthermore, the borane acts equally a lewisAnti-Markovnikov acid by accepting two electrons in its empty p orbital from an alkene that is electron rich. This process allows boron to have an electron octet. A very interesting characteristic of this procedure is that it does not require whatever activation by a catalyst. The Anti-MarkovnikovHydroboration mechanism has the elements of both hydrogenation and electrophilic addition and information technology is a stereospecific ( syn addition), pregnant that the hydroboration takes identify on the same face of the double bond, this leads cis stereochemistry.
Introduction
Hydroboration-oxidation of alkenes has been a very valuable laboratory method for the stereoselectivity and regioselectivity of alkenes. An Additional feature of this reaction is that it occurs without rearrangement.
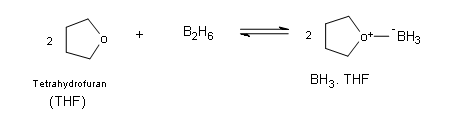
The Borane Circuitous
Outset off information technology is very imporatnt to understand little bit virtually the structure and the properties of the borane molecule. Borane exists naturally equally a very toxic gas and it exists as dimer of the general formula B2H6 (diborane). Additionally, the dimer BiiH6ignites spontaneously in air. Borane is commercially available in ether and tetrahydrofuran (THF), in these solutions the borane tin be every bit a lewis acid-base of operations circuitous, which allows boron to have an electron octet.
\[ 2BH_3 \rightarrow B_2H_6\]
The Mechanism
Stride #1
- Part #1: Hydroboration of the alkene. In this beginning footstep the addittion of the borane to the alkene is initiated and prceeds every bit a concerted reaction because bond breaking and bail formation occurs at the aforementioned time. This role consists of the vacant 2p orbital of the boron electrophile pairing with the electron pair of the ? bondof the nucleophile.
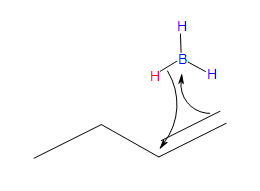
Transition state
.bmp?revision=1&size=bestfit&width=441&height=242)
* Annotation that a carbocation is not formed. Therefore, no rearrangement takes identify .
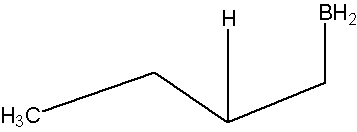
- Function #2: The Anti Markovnikov addition of Boron. The boron adds to the less substituted carbon of the alkene, which and then places the hydrogen on the more substituted carbon. Both, the boron and the hydrogen add simultaneously on the same face of the double bond (syn addition).
Oxidation of the Trialkylborane past Hydrogen Peroxide
Stride #2
- Part #ane: the outset office of this mechanism deals with the donation of a pair of electrons from the hydrogen peroxide ion. the hydrogen peroxide is the nucleophile in this reaction because it is the electron donor to the newly formed trialkylborane that resulted from hydroboration.

EpoxidationEpoxidation .bmp?revision=1&size=bestfit&width=696&height=239)
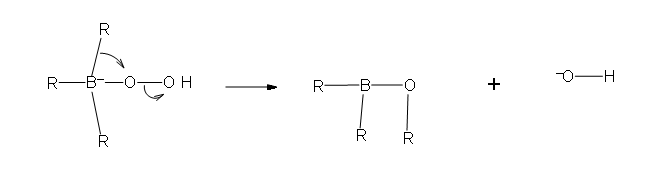
- Role ii: In this second part of the machinery, a rearrangement of an R group with its pair of bonding electrons to an side by side oxygen results in the removal of a hydroxide ion.
Two more than of these reactions with hydroperoxide volition occur in order give a trialkylborate
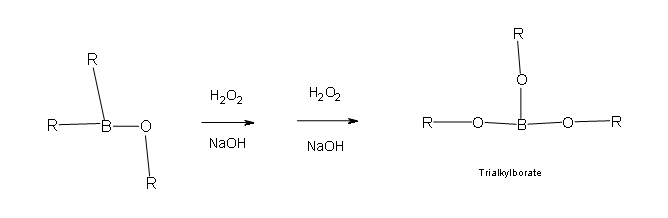
- Part 3: This is the final part of the Oxidation process. In this part the trialkylborate reacts with aqueous NaOH to give the alcohol and sodium borate.

If yous need additional visuals to aid y'all in agreement the mechanism, click on the outside links provided here that will take you to other pages and media that are very helpful too.
References
- Vollhardt, Peter, and Neil Shore. Organic Chemistry: Structure and Part. fifth. New York: W.H. Freeman and Company, 2007.
- Foote, Southward. Christopher, and William H. Brown. Organic Chemistry. fifth. Belmont, CA: Brooks/Cole Cengage Learning, 2005.
- Bruice, Paula Yurkanis. Oragnic Chemical science. 5th. CA. Prentice Hall, 2006.
- Bergbreiter E. David , and David P. Rainville. Stereochemistry of hydroboration-oxidation of concluding alkenes. J. Org. Chem., 1976, 41 (18), pp 3031–3033
- Ilich, Predrag-Peter; Rickertsen, Lucas S., and Becker Erienne. Polar Addition to C=C Grouping: Why Is Anti-Markovnikov Hydroboration-Oxidation of Alkenes Not "Anti-"? Journal of Chemical Didactics., 2006, v83, n11, pg 1681-1685
Problems
What are the products of these following reactions?
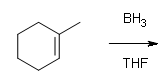
#1.
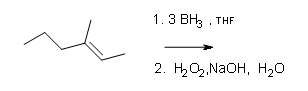
#2.
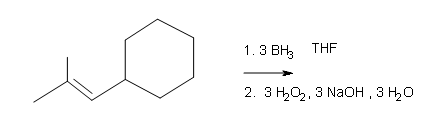
#iii.
Describe the structural formulas for the alcohols that event from hydroboration-oxidation of the alkenes shown.
#four.
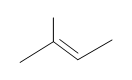
#v. (E)-3-methyl-2-pentene
If yous need clarification or a reminder on the nomenclature of alkenes refer to the link beneath on naming the alkenes.
Answers
#ane.
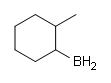
#ii.
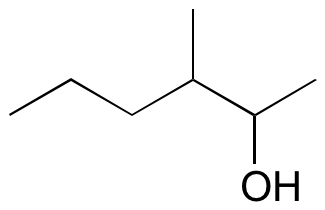
#three.
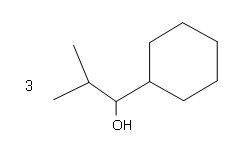
#four.
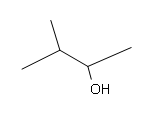
#5.
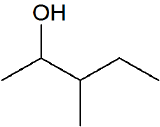
Contributors
- Gilbert Torres (UCD)
Source: https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Supplemental_Modules_(Organic_Chemistry)/Alkenes/Reactivity_of_Alkenes/Hydroboration-Oxidation_of_Alkenes
0 Response to "what alkene is required to make 3-methyl-1-butanol using the hydroboration-oxidation reaction?"
Post a Comment